Cathleen Zeymer
Artificial Metalloenzymes and Photoenzymes

Artificial metalloenzymes are powerful catalysts that combine the reactivity of transition metal complexes with the selectivity of proteins. They are generated by rational design and optimized for specific chemical reactions by directed evolution, a technique that mimics natural selection in the laboratory. In addition to applications in biocatalysis, the detailed analysis of molecular structures and mechanisms is one of the lab’s key objectives. Furthermore, we make use of photoexcitation as a catalytic tool to trigger alternative activities in both natural and de novo designed enzymes. Our efforts in photobiocatalysis focus on enabling and controlling light-induced radical reactions in the chiral environment of the proteins.
Key publications:
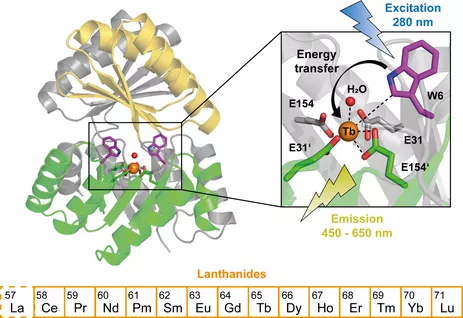
Tight and specific lanthanide binding in a de novo TIM barrel with a large internal cavity designed by symmetric domain fusion, S. J. Caldwell, I. C. Haydon, N. Piperidou, P. S. Huang, M. J. Bick, H. S. Sjöström, D. Hilvert, D. Baker,* C. Zeymer,* Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 30362–30369.
Directed evolution of protein catalysts, C. Zeymer, D. Hilvert,* Annu. Rev. Biochem. 2018, 87, 131–157.
Optimization of Enzyme Mechanism along the Evolutionary Trajectory of a Computationally Designed (Retro-)Aldolase, C. Zeymer, R. Zschoche, D. Hilvert,* J. Am. Chem. Soc. 2017, 139, 12541–12549.